BioSynchronicity is an agile company with a well-balanced and experienced professional team having in-depth business expertise in technology development, healthcare delivery, and international trade.


Keep Our Communities Safe

half-century have all been caused by zoonotic viruses, meaning
they are transmitted from animals to people.
detect infection from emergent viruses is very urgently needed.
Who we are
Research & Development
State-of-the-art R&D targeting testing solutions for the present and future needs of healthcare.
Quality Manufacturing
Quality control and quality assurance guaranteed. All components are manufactured under ISO-13485 certified protocols with superior risk mitigation protocols, supervision, and process oversight.
Global Impact
Professional distribution partners and healthcare professionals promoting meaningful and impactful solutions in each market where we are active globally.
Products
Stayin' Alive™
Insta-Test™
Stayin' Alive™ Insta-Test™
Page Under Construction

Testing to Prevent the Spread of Infectious Diseases
Medical experts have stated repeatedly that frequent testing with affordable and reliable rapid antigen diagnostic tests for infectious diseases is a necessary component of an effective integrated strategy to mitigate the transmission of viral and bacterial infections.
A Ready, Willing, and Able Team
The BioSynchronicity team is ready, willing, and able to provide reliable, affordable, and highly accurate in vitro diagnostic tests to clients globally.
Manufacturing
Components are manufactured under strict supervision and quality control/assurance protocols. Good workmanship is guaranteed in every step of the manufacturing process.
Packaging
Packaging will be adapted to the customer’s needs. Packaging can be customized in appropriate languages and include symbols or certification required by local regulatory bodies.
Quality Control
We ensure manufacturing of our products is conducted under strict certified ISO 13485 protocols.
Performance Testing
Analytical testing of our products is conducted by a highly accredited and respected lab in Rockville, Maryland, whose CEO is a former President of the Washington Academy of Science.
Certification
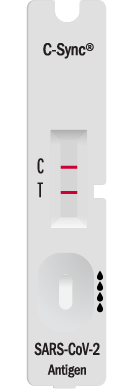
The BioSynchronicity C-Sync™ COVID-19 Antigen Test received its FDA Emergency Use Authorization (EUA) on March 23, 2023. The EUA letter of authorization from the FDA allows us to conduct sales in the United States at the point of care.
We have also achieved the CE Mark certification for the European Union and have registered in the European Union with the Ministry of Health of The Netherlands. Additional countries in MENA and Africa recongize the authority of the CE certification. A Free Sales Certificate has been successfully issued for the UAE and validated by the UAE Embassy in The Netherlands.
We are able to sell products throughout the USA, Europe, MENA and Africa.

FDA EUA (EUA220346)

CE Certification